
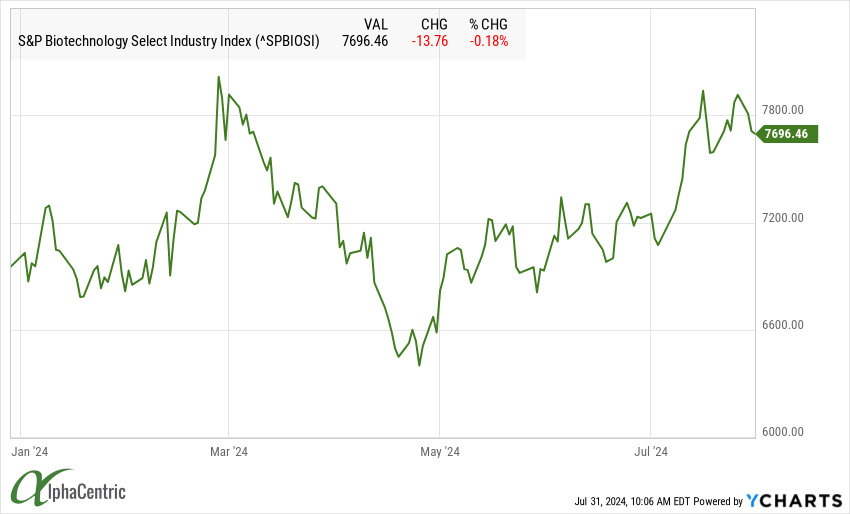
The S&P Select Biotech Index remained at the high end of its recent trading range as the focus increasingly turns to company updates in the ongoing earning season.
Big pharmas have generally been rallying on strong results and growing confidence that IRA price controls may be less draconian than feared. Case in point was Bristol Myers Squibb's (BMY) management commentary on the recent earnings call that disclosed "we've seen the final price, we're increasingly confident in our ability to navigate the impact of IRA on Eliquis." BMY expects CMS to publish the price either on or before September 1.
Analysts are now generally expecting 15-30% discounts from current net brand pricing, a departure from the >50% in early CBO projections that provided the basis of the claim that overall savings would be $100+ billion
M&A:
Collegium (COLL) announced a deal to acquire Ironshore Therapeutics (private) for $525mn in cash focused on lead asset JORNAY PM (methylphenidate HCl), a central nervous system (CNS) stimulant prescription medicine for the treatment of attention deficit hyperactivity disorder (ADHD)
Regulatory:
Biogen (BIIB) announced European regulators recommended against approval of Alzheimer's therapy Leqembi due to a poor risk / benefit profile given the risk of ARIA and muted clinical benefit. The company plans to seek re-examination
Clinical:
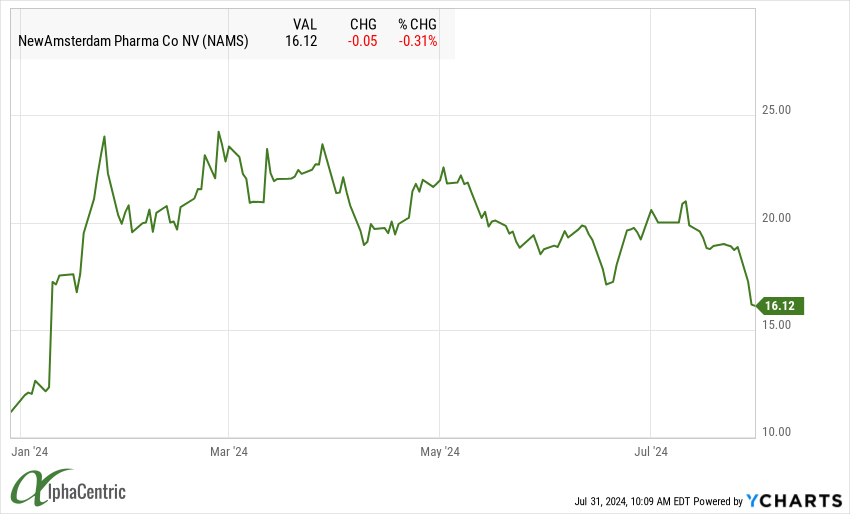
New Amsterdam Pharmaceuticals (NAMS) shares fell after announcing pivotal BROOKLYN trial of obicetrapib in patients with heterozygous familial hypercholesterolemia lowered LDL by 36.5% at day 84, below heightened street expectations for a >40-45% reduction
Sangamo Therapeutics (SGMO) reported positive P3 data for Pfizer (PFE) partnered gene therapy giroctocogene fitelparvovec in Hemophilia A. Data appears competitive with competitor BioMarin's Roctavian (mean ABR 1.24 vs. 2.6 Roctavian), but commercial uptake remains an overhang with very few patients receiving Roctavian since approval
Sage Therapeutics (SAGE) was under pressure after disclosing SAGE-234 did not demonstrate any statistically significant from placebo in the Phase 2 KINETIC 2 study in patients with essential tremor (ET). Development of SAGE-234 in ET will be discontinued
Corporate Updates & Earnings:
Edwards Lifesciences (EW) crashed 30% its largest drawdown since 2000 on a Q2 miss. Revenue was ~1% light, but 2H 2024 TAVR growth was lowered to 5-7% from 8-10%
Dexcom (DXCM) was down >30% on ~3% revenue miss and lowered 2024 revenue guidance of $4-4.05 billion down from $4.2-4.35 billion (street $4.36 billion)
Argenx (ARGX) reported 2Q vyvgart revenue ahead of the street ($477 million vs street $425 million), swinging the bottom line to be profitable for the first time
Abbvie (ABBV) raised 2024 EPS guidance to $10.71-$10.91 representing a 10 cent increase over the prior midpoint. Strong anti-inflammatory sales of Skyrizi and Rinvoq offset declining Humira
Bristol Myers (BMY) reported 2Q revenue ahead by ~6% and EPS by 28%, 2024 guidance raised by 20 cents at the midpoint
Sanofi (SNY) reported a quarterly beat and raise with revenue ~4% ahead. Notably, Dupixent grew by 29%, nearly 5% ahead of the street
Roche (RHHBY) raised 2024 EPS guidance to high-single digit growth from mid-single digit growth. The CEO expressed very high chance for obesity asset success
Bausch Health (BHC) fell >20% on a false news report that the company was preparing for a Chapter 11 bankruptcy filing. The company immediately denied the report, but failed to reverse the move
Viking Therapeutics (VKTX) announced they will move obesity candidate VK2735 into a phase 3 registration trial, earlier then expected
Ipsen Pharmaceuticals (IPN) and Day One Biopharmaceuticals (DAWN) announced an ex-US licensing agreement for Ojmeda, the first FDA approved therapy for pLGG, for $111 million upfront, $350 million in milestones and a double digit royalty
Geron Corporation (GERN) was under pressure after announcing the CCO Anil Kapur was leaving the company amidst the launch of Rytelo. A search for a replacement is underway





Comments